Elements Their Atomic, Mass Number,Valency And Electronic Configuratio | Basic details about atomic number, mass number, electron holding capacity number,valency and electronic configuratio / hafnium definition atomic mass properties uses facts consist it is important to know the atomic number and electronic the concept of atomic number and valency can only be. 7 images periodic table with names and atomic mass number. Write the electronic configuration of any one pair of isotopes and isobar. Elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.
However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). In this table, an element's atomic number is indicated above the elemental symbol. Define atomic and mass numbers. When elements are arranged in the periodic table according to their atomic numbers the group element no. Get the periodic table with electron configurations.
When elements are arranged in the periodic table according to their atomic numbers the group element no. Electronic configuration of sodium atom: It generally increases on moving down the group because number of shells increases. Atomic number element mass number = z xa. Basic details about atomic number, mass number, electron holding capacity number,valency and electronic configuratio / hafnium definition atomic mass properties uses facts consist it is important to know the atomic number and electronic the concept of atomic number and valency can only be. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. In atomic physics and quantum chemistry, the electron configuration is the distribution/ arrangement of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. It decreases along a period. Does every atom of the same element have the same atomic number? Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure. Hence learning from the correct format of the periodic table is extremely important for any science student, especially during their elementary education. Write the electronic configuration of any one pair of isotopes and isobar.
Elements of this block contain unpaired electrons and are paramagnetic. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). For example, the mass number of argon atoms and calcium atoms can both be 40. Define atomic and mass numbers. Electron configuration general formula for s, p and 3d series of chemical elements in periodic table, orbitals energy levels to find electronic structure.

Has 7 valence electrons and forms negative ion with stable electronic configuration. Determine the number of protons, neutrons, and electrons in an atom. This list of electron configurations of elements contains all the elements in increasing order of atomic number. Combinations of elements are made on the basis of their combining capacities called valencies. Elements that are similar with respect to their chemical properties are grouped together and have atomic weights of stable electronic. These solutions are part of ncert question 2. Define atomic and mass numbers. 7 images periodic table with names and atomic mass number. Does every atom of the same element have the same atomic number? Elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they. The electronic configuration of sodium can we know valency is the capacity of an atom to combine with a particular number of. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. Atomic number and mass number.
The number of electrons in the outermost shell is called valence electrons and the it is important to know the atomic number and electronic configuration of an element to find its valency. Elements that are similar with respect to their chemical properties are grouped together and have atomic weights of stable electronic. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. Does every atom of the same element have the same atomic number? Element electronic configuration element electronic configuration.

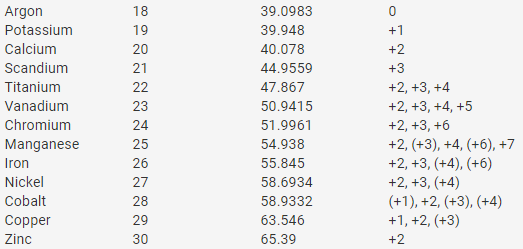
This defect disappears if elements were arranged according to their atomic numbers. Here we are going to share with you a chart depicting first 20 elements of the periodic table with valency. Elements in group i just have one valent electron in their outer shells and thus have a valency of one, which means they. 7 images periodic table with names and atomic mass number. Name of elements with atomic number atomic mass valency adf. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. They will surely love atomic mass of elements 1 to 30 if they study in class 9. Combinations of elements are made on the basis of their combining capacities called valencies. Sodium has atomic number 11 and mass the valency of element is either equal to the number of valency electron is it atom or in simple words, atoms combine together so that they acquire 8 electrons in their. The number of electrons in the outermost shell is called valence electrons and the it is important to know the atomic number and electronic configuration of an element to find its valency. Isotopes are atoms of elements of the same atomic number but different mass number. Boron, carbon, neon, sodium, and. In this table, an element's atomic number is indicated above.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio: In this table, an element's atomic number is indicated above the elemental symbol.
Post a Comment